Date: 2022-07-12
hits: 562
Table of contents
—Direct ELISA Vs indirect ELISA
Introduction
1)Direct ELISA definition
2)Indirect ELISA definition
—What is the difference between direct and indirect ELISA?
1)Direct ELISA
-Principle
-Feature-Advantages
-Disadvantages
-Indirect ELISA
-Principle
-Feature
-Advantages
-Disadvantages
—Direct Protocol VS InDirect ELISA Protocol Difference
Protocol Steps
—Similarities Between Direct and Indirect ELISA
—Why use indirect ELISA instead of direct ELISA?
—Which type of ELISA should I use?
Direct ELISA vs Indirect ELISA
Direct ELISA Introduction
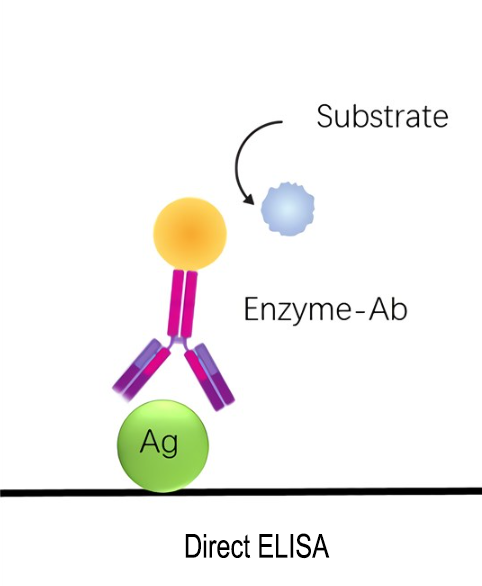
A direct ELISA (enzyme-linked immunosorbent assay) is an immunosorbent test that uses plates to detect and quantify a particular analyte (such as antigens, antibodies, proteins, hormones, peptides, etc.) from a complicated biological sample. The easiest and quickest to use of the four ELISA formats is direct ELISA, yet there are several drawbacks to this technique.
In a direct ELISA, the antigen is directly adsorbed onto the surface of a multi-well microtiter plate, such as a 96-well polystyrene plate, and is then complexed with a primary antibody that has been enzyme-labeled and is specific for the antigen.An absorbance microplate reader or spectrophotometer is used to detect the visual colorimetric output produced by the conjugated primary antibody after it has bound to the antigen and catalyzed a reaction with its corresponding substrate. For qualitative and quantitative antigen detection in test samples, antibody screening, and epitope mapping, direct ELISAs are appropriate.
Indirect ELISA introduction
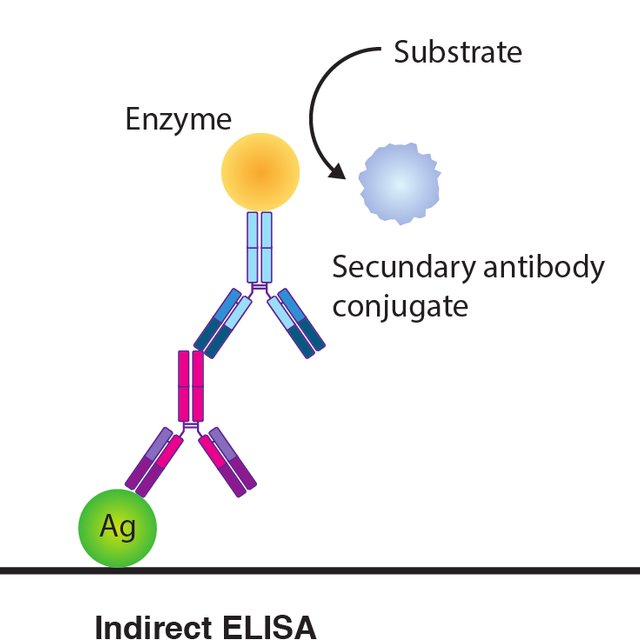
Indirect ELISA is a two-step detection procedure where a primary antibody specific for the antigen binds to the target and a tagged secondary antibody against the primary antibody's host species binds to the primary antibody to detect the antigen.
What is the difference between direct and indirect ELISA?
1)Direct ELISA
Principle:
Immobilize the antibody or antigen on an enzyme-labeled plate, then use the enzyme-labeled antibody or antigen to directly detect it.
Feature:
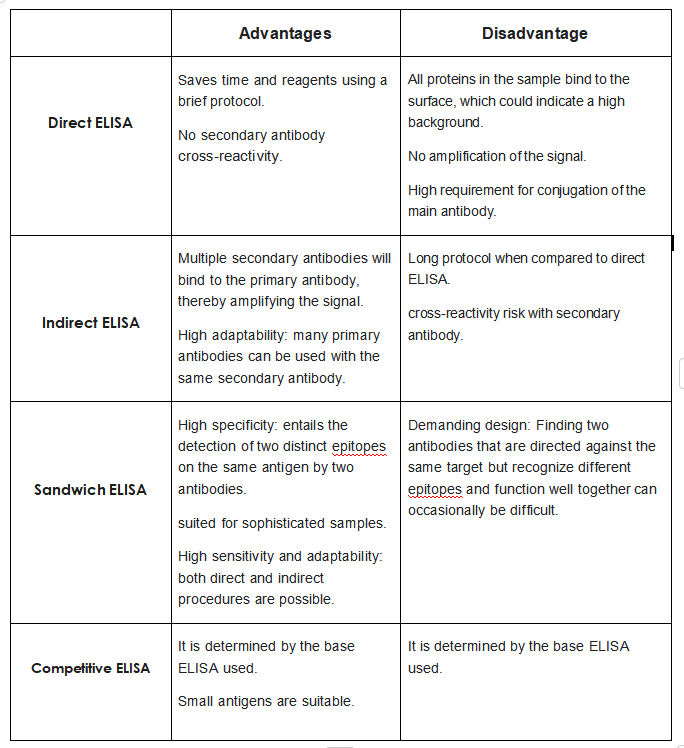
Compared to other types of ELISA assays, direct ELISA has fewer steps, is faster, does not require the use of secondary antibodies, avoids cross-reactivity and is less prone to error in the assay results. However, because the antigen in a direct ELISA is not specifically fixed, target proteins and other impurities in the sample will bind to the ELISA plate and the background of the experiment will be higher. Moreover, direct ELISA requires a primary antibody for each target protein that binds specifically to it, making the experiment less flexible. In addition, as no secondary antibody is used, the signal is not amplified, reducing the sensitivity of the assay.
Advantages:
• Quick because only one antibody and fewer steps are used.
• Cross-reactivity of secondary antibody is eliminated.
Disadvantages:
• Immunoreactivity of the primary antibody might be adversely affected by labeling with enzymes or
tags.
• Labeling primary antibodies for each specific ELISA system is time-consuming and expensive.
• No flexibility in choice of primary antibody label from one experiment to another.
• Minimal signal amplification.
2)Indirect ELISA
Principle
The antigen is initially coupled to the ELISA plate, and then in two phases the antigen is detected: first by adding the detection antibody to bind specifically to the antigen, then by adding the enzyme-labelled secondary antibody for detection and color development using the substrate.
Feature
Compared to direct ELISAs, indirect ELISAs use enzyme-labelled secondary antibodies, which offer greater sensitivity and require fewer labelled antibodies, making them more economical. Indirect ELISAs also offer greater flexibility as different primary antibodies can be used with a single labelled secondary antibody. The disadvantages of indirect ELISA assays are the possibility of cross-reactivity (the enzyme-labelled secondary antibody binds directly to the antigen), which may increase the background, and the additional step of incubating the secondary antibody compared to direct ELISA, which increases the cycle time of the assay.
Advantages
• A wide variety of labeled secondary antibodies are available commercially.
• Versatile because many primary antibodies can be made in one species and the same labeled
secondary antibody can be used for detection.
• Maximum immunoreactivity of the primary antibody is retained because it is not labeled.
• Sensitivity is increased because each primary antibody contains several epitopes that can be bound by
the labeled secondary antibody, allowing for signal amplification.
Disadvantages
• Cross-reactivity might occur with the secondary antibody, resulting in nonspecific signal.
• An extra incubation step is required in the procedure
Direct Protocol VS Indirect ELISA Protocol Difference
• Direct ELISA Protocol
The procedure for a direct ELISA test is as follows:
Each well of a microtiter plate (often 96-well plates) is filled with a buffered solution of the antigen to be tested for, where it is given time to interact with charges and attach to the plastic.
Each well receives an addition of a nonreacting protein solution, such as bovine serum albumin or casein, to cover any plastic surfaces that the antigen failed to coat.
The primary antibody is then added, along with a conjugated enzyme that selectively binds to the test antigen covering the well.
Then, an enzyme substrate is included. This substrate frequently changes color when it interacts with the enzyme.
The higher the concentration of the primary antibody present in the serum, the stronger the color change. Often, a spectrometer is used to give quantitative values for color strength.
In the literature and on websites, the terms "indirect ELISA" and "direct ELISA" are used and understood differently depending on the context of the experiment.
When analyzing the presence of an antigen,"indirect ELISA" refers to an ELISA in which the antigen is bound by the primary antibody and then detected by a labeled secondary antibody,
whereas "direct ELISA" refers to an ELISA in which only a labelled primary antibody is used. The latter situation clearly distinguishes a sandwich ELISA from an indirect ELISA.
When the "primary" antibody is of interest, as in immunization analyses, this antibody is directly detected by the secondary antibody, and the phrase "indirect ELISA" refers to a method in which the secondary antibody is not used.
• Indirect ELISA Protocol
Indirect ELISA test method does not use the traditional wells. This test leaves the antigens suspended in the test fluid.
Unlabeled antibody is incubated with its antigen (sample) present.
The antibodies are given enough time to attach to the antigens throughout the incubation phase.
After that, the sample is run through the Scavenger container. A test tube or a specially made flow through channel can serve as this. "Scavenger Antigens" are attached to the surface of the Scavenger container or channel. These can be the same as the primary antigens or sufficiently similar to them for the free antibodies to bind.
It is necessary for the scavenger container to have enough surface area and time for the scavenger antigens to bind to all the extra antibodies added to the sample.
The sample is run through a detector after having been modified to include the tagged and bound antibodies. This tool may be a flow cytometer or another tool that activates the tags and records the response.
This assay enables simultaneous tagging and counting of many antigens. This makes it possible to distinguish between distinct bacterial strains using two (or more) different color markers. A cell is that particular strain if both tags are visible on it. If there is just one there, it is not.
This test cannot be performed using a microtiter plate and is often conducted one test at a time. Typically, the required equipment is simpler and portable.
Similarities Between Direct and Indirect ELISA
The two ELISA procedures are direct and indirect ELISA, in which the antigen of interest is directly affixed to the plate.Both methods involve detecting the presence of a particular protein molecule in a sample. This protein molecule is in direct contact with the plate.
Both approaches employ 96-well plates or 8-well strip tubes. These plates and tubes are both composed of polystyrene. The 96-well plates have ample of room for determining the correct dilution for the reaction. Meanwhile, 8-well strip tubes are preferable for fine-tuned assays.
Why use indirect ELISA instead of direct ELISA?
The indirect ELISA method is more cost-effective than the direct ELISA because it requires fewer labeled antibodies and has a higher sensitivity because more than one labeled secondary antibody can bind the primary antibody.ELISAs tend to be the most sensitive immunoassays due to the binding characteristics of the antibodies and the amplification or different read-out systems used. Sample volumes can also be adjusted when you have a very low abundant protein. As discussed above, indirect ELISAs allow for the amplification of signal by using a secondary antibody. Other amplification systems can also be used in ELISAs to make High Sensitivity ELISA Kits, where an additional amplification step is used to increase the sensitivity.
Which type of ELISA should I use?